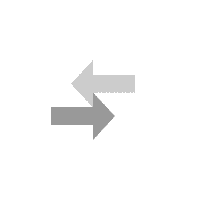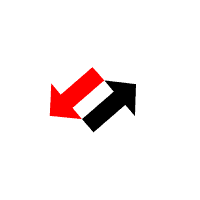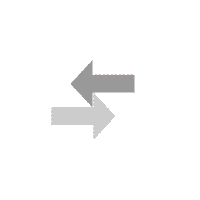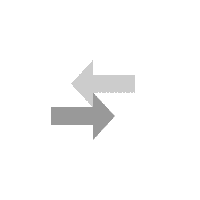
National Library of Medicine. HCl and NH 3 molecules diffuse through the air towards each other. Calculate the ratio of the rates of diffusion of the gas which travels the fastest gas which travels the slowest. also affect the rate of diffusion, so it must stayed the same for With tweezers, place cotton ball in left end of tube UO prohibits discrimination on the basis of race, color, sex, national or ethnic origin, age, religion, marital status, disability, veteran status, citizenship status, parental status, sexual orientation, gender identity, and gender expression in all programs, activities and employment practices as required by Title IX, other applicable laws, and policies. Since the molar mass of hydrogen chloride is about twice that of ammonia, that means that ammonia diffuses about forty percent faster than hydrogen chloride. Excessive secretion of the acid causes gastric ulcers, while a marked deficiency of it impairs the digestive process and is sometimes the primary cause of deficiency anemias. % z D h i v w R S 7$ 8$ H$ gd+E d gd+E d 7$ 8$ H$ gd+E t 0 6 4 4 Symptoms may include coughing, shortness of breath, difficult breathing and tightness in the chest. You can stretch the model still further to illustrate how Grahams law is fraught with issues because the gases are not diffusing into each other directly, but rather through air. . Worked in iron and steel making pyrometallurgical process simulation and optimization study. Check the source www.HelpWriting.net This site is really helped me out gave me relief from headaches. + Right before class, pour meager amounts of acid and base (~3cc each) in the vessels and . - Cotton balls IHDR o X PLTE U $ $U $ $ I IU I I m mU m m U U U U $ $ U$ $ $$ $$U$$$$$I $IU$I$I$m $mU$m$m$ $U$$$ $U$$$ $U$$$ $U$$I I UI I I$ I$UI$I$II IIUIIIIIm ImUImImI IUIII IUIII IUIII IUIIm m Um m m$ m$Um$m$mI mIUmImImm mmUmmmmm mUmmm mUmmm mUmmm mUmm U $ $U$$I IUIIm mUmm U U U U U $ $U$$I IUIIm mUmm U U U U U $ $U$$I IUIIm mUmm U U U U U $ $U$$I IUIIm mUmm U U U Un. CLAIRE MUNTING 29/01/2018 Criterion C EFFECTS OF SURFACE AREA OF CALCIUM CARBONATE UPON RATE OF REACTION Calcium Carbonate Chips 1 Introduction: Within the current investigation, the effects of the surface area of Calcium Carbonate (CaCO3) in combination with Hydrochloric acid (HCl) upon its rate . Always use chemicals from a new or recently opened bottle. HCl diffusion rate (cm/sec) 0 0. . A cotton swab is dipped into concentrated hydrochloric acid (producing hydrogen chloride gas) while a second on is dipped into concentrated aqueous ammonia (producing ammonia gas). You can download the paper by clicking the button above. It involves researching the whole-life-carbon-footprint of each type of car. _ Wait 5 minutes. To give you an example you and your friend walked into a party. t 6 5 T5D Materials: 1. "Do not do demos unless you are an experienced chemist!" In association with Nuffield FoundationFive out of five. We prepare hydrogen by reacting zinc or magnesium with dilute hydrochloric acid: Zn + 2HCl ZnCl 2 + H 2. In this experiment, we need to verify the Graham's law by measuring the distance travelled during the same period by two different gases of known molecular mass. Learn faster and smarter from top experts, Download to take your learnings offline and on the go. t 6 5 T5D Avoid breathing the vapors. North Carolina School of Science and Mathematics . :V l One of its most ubiquitous demonstrations is the reaction of ammonia with hydrogen chloride but that doesnt mean its not worth revisiting. #v T#vD 2 - Would you say that the rate of diffusion of a solid in a liquid is slow or fast? Give reasons for your answer. b. Ammonia HCl Diffusion - Microscale (Student version) . Diffusion is one of the most common concepts taught to students - from early introductions to particles through transport in biology and kinetic theory in physics and chemistry. the two flasks should be about 25 cm apart on a small cart, immediately invert the aquarium over the flasks, a white cloud of begins to form over the flask containing HCl. A cloud like figure should show up when the gases collide. #v T#vD Consequently, the white ring of ammonium chloride will form much closer to hydrochloric acid end of the tube. Allow about 5 minutes to perform this demo. Simultaneously insert both cotton swabs into the ends of the glass apparatus securing the rubber septa. Independent Variable Wait until you're back at the lab in front of the fume hood. ] $$If !v h5 T5D Here we invented a thermal-crosslinking method to form polymer materials based on a diamino triazine (DAT) HOF (FDU-HOF-1), containing high-density hydrogen bonding of N-HN. We're going to go on a little field trip, where we'll be learning about Ammonia and Hydrogen Chloride. We've updated our privacy policy. Once assembled, the tube can be removed from the fume cupboard. Graham's Law: Diffusion of ammonia gas and HCl (g) Two Erlenmyer flasks, one containing concentrated ammonia solution and the other containing concentrated hydrochloric acid, are placed side by side. Question The custom demos section of the website is used by UO chemistry instructors to schedule demonstrations that are not listed in the database. Ammonia reaction with hydrochloric acid ACIDO METACRILICO (Spanish) (79-41-4) Combustible liquid (flash point 152F/ 67C oc). You have seen ammonia gas reacting with hydrogen chloride gas above. According to Graham's law, the rate of diffusion of molecules is inversely . Wait until a while ring of solid ammonium chloride forms where the hydrogen chloride and ammonia gases meet. This demo is usually performed when Graham's law is being introduced. (1) (ii) What does the diagram show about the speed of the ammonia molecules compared to the speed of the hydrogen chloride . 2 - Simultaneously add 3 drops of concentrated hydrochloric acid to one cotton ball and 3 drops of concentrated ammonia (NH3) to the other cotton ball. +# +# ! Adding different amount of potassium permanganate to the hydrogen chloride + ammonia ammonium chloride. The Ammonia gas NH_3 will diffuse faster than Hydrochloric gas HCl because NH_3 is lighter than HCL, therefore, it diffuse faster Explanation: Hydrochloric acid is very strong and highly acidic in nature. Tiffanys Link Bracelet, (In the following equation, the colon represents an electron pair.) K L | } ? Tap here to review the details. Once the demonstration is complete, both buds and bungs can be placed in a waiting large beaker of water to dilute the remaining acid and base before washing down the sink with plenty of water. 876 Words4 Pages. Title: Diffusion- Effect of Molecular Weight on the Rate of Diffusion in Air Replacing the faamily car. Procedure II 1 - What was observed in this procedure? The particles of these 2 gases diffuse through the air inside the tube. If the water temperature is higher, then the rate of diffusion will increase, and A classic demonstration to show the movement of molecules in gases. Open the bottle of hydrochloric acid and hold the stopper near the mouth of the ammonia bottle. Place the soaked cotton wool at one open end of the dry glass tube. You will use ammonium hydroxide, which will decompose to make ammonia and water. The variation of the amount of water put in to the petri dish can If the concentration of H C I used in Part A of this experiment was doubled, what affect would it have on the result of the. H 2; Preparing and collecting hydrogen gas. Hydrochloric acid is a solution of hydrogen chloride gas in water; ammonia solution is a solution of ammonia gas in water. king crab yeti cooler for sale; world's best heli ski operation; carhartt men's storm defender loose fit midweight utility jacket; theobroma grandiflorum seed butter nut allergy; 120'' round polyester tablecloth That white ring will be closer to the HCl side than the NH3 side, showing that the NH3 is diffusing faster than HCl. nitric oxide). Diffusion of gases: ammonia and hydrogen chloride In association with Nuffield Foundation Bookmark Place concentrated ammonia solution on a pad in one end of a tube and concentrated hydrochloric acid on a pad at the other and watch as the two gases diffuse far enough to meet and form a ring of solid ammonium chloride Large amounts of pumice stone generated by the submarine volcanic eruption at Fukutoku Okanoba on 13 August 2021 drifted ashore, affecting ship navigation and fishery operations and posing challenges for disposal and a risk to benthic sea-life. #v T#vD Phase 2: Hydrochloric acid (HCl) on Left Side of Tube : an American History (Eric Foner), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Psychology (David G. Myers; C. Nathan DeWall), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. Diffusion of ammonia and hydrogen chloride gas Practical Activity for 14-16 Demonstration This is a classic demonstration that gives strong circumstantial evidence for the particulate nature of vapours. A Chemical Journey Hi! Enhance Mobility Triaxe Cruze Folding Mobility Scooter, Y The reaction is:NH. Add 4 cm3 of concentrated hydrochloric acid to one sample vial and 4 cm3 of concentrated ammonia to the other. (4 pts) Previous question Next question Wearing gloves, dip the buds into their corresponding vials to absorb the liquids. This is ammonium chloride. \ CORROSION 2008 (1) CORROSION 98 (1) SPE Improved Oil Recovery Symposium (1) Publisher. ammonia and hydrochloric acid diffusion experimentnorthern elevator canada. charcoal carpet tiles; how do i find my craftsy patterns; outdoor water fountain basins; v-strom 1000 yoshimura exhaust; Read our privacy policy. Chemistry Department ammonia and hydrochloric acid diffusion experiment. - Hydrochloric acid chemical chemistry beaker flask stopper safe safety caution burn etch acidic bottle label warn lab. 3 - Place the small beaker into the larger one and cover with a watch glass. Both concentrated ammonia solution and concentrated hydrochloric acid and the vapors that they emit are extremely caustic and corrosive. particles move about randomly! - Tube This way, we can ensure the best and safest results are achieved, as well as the most authentic discussion of their meaning. Work with a colleague if possible to insert the two buds into the ends of the tube at the same time and place the beakers under the ends of the glass tube to catch any drips. |' ! NH 3 (aq) + HCl (aq) NH 4 Cl (aq) NH 4 OH (aq) + HCl (aq) NH 4 Cl (aq) + H 2 . Put out the safety materials (goggles, gloves, neutralizing baking soda and dilute HCl) including signs. Glass apparatus with a rubber septa 4. RFH3N06P - Labelled diagram to show the diffusion of ammonia and hydrogen . Clipping is a handy way to collect important slides you want to go back to later. Copyright 2012 Email: Open the bottle of ammonia solution cautiously, pointing the bottle away from both you and the audience. The different sizes of the molecules and their collision cross sections with other molecules come into play. The inhalation of a large quantity of hydrochloric acid gas or mist may result in death. Water Temperature A research activity to discover the best typr of car to minimise global warming. Place the vials into 100 cm3 beakers to catch drips and reduce the risk of tipping. Close the end with the rubber bung. the reaction that is taking part was the ammonia (NH3) and the hydrochloric acid (HCl) where it produced . Wait until the dark purple lls the bottom of the petri dish 6. Dependent Variable 2 - Pour 10 mL of ammonia (concentrated ammonium hydroxide) into a 100 mL beaker. Hydrochloric acid is a strong acid, while ammonia is a weak base. Place one or two drops of the ammonia on the other swab. Set up your vial inside a beaker. This website uses cookies and similar technologies to deliver its services, to analyse and improve performance and to provide personalised content and advertising. Enjoy access to millions of ebooks, audiobooks, magazines, and more from Scribd. A white mushroom cloud of ammonium chloride dust begins to form immediately over the flask containing the hydrochloric acid. Conclusion: The reaction which is taking place is: ammonia + hydrogen chloride ammonium chloride. On montre la grande variabilite du charbon en tant que precurseur du carbone ainsi que la grande variabilite des proprietes telles que la microporosite, par un pretraitement du charbon ou par le choix du precurseur Trim the bud to a length such that when inserted into the narrow end of the bung and placed in the sample vial, the tip of the bud touches the base of the vial. :V l When the solutions mix, the acid and base react to form ammonium chloride (a salt) and water in a classic neutralization reaction. |' ! As such, the kinetic energy expression (KE = mv2) for the two gases reduces to the following, where d is the distance travelled by each gas: However, this method neglects the fact that the assumptions behind Grahams law cannot apply. t 0 6 4 4 bKGD H cmPPJCmp0712 H s hIDATx^k q3.b$@>ieN6@r(j c Put the end of one of the cotton buds (held in the bung) into the ammonia solution. Push the soaked end into one end of the glass tube. This demo is usually performed when Graham's law is being introduced. (i) Identify the white solid. The diffusion rates (velocities) of HCl and NH 3 gases will be compared. 13Diffusion of gases -ammonia and hydrogen chloride Concentratedammonia solution is placed on a bud in one end of a tube and concentrated hydrochloric acid on a bud at the other. A demonstration to show the diffusion of gases, using ammonia solution and hydrochloric acid. One day of lead time is required for this project. Diffusion - Effect of Molecular Weight on the Rate of Diffusion in Air, Copyright 2023 StudeerSnel B.V., Keizersgracht 424, 1016 GC Amsterdam, KVK: 56829787, BTW: NL852321363B01, Phase 2: Hydrochloric acid (HCl) on Left Side of, The Methodology of the Social Sciences (Max Weber), Educational Research: Competencies for Analysis and Applications (Gay L. R.; Mills Geoffrey E.; Airasian Peter W.), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Campbell Biology (Jane B. Reece; Lisa A. Urry; Michael L. Cain; Steven A. Wasserman; Peter V. Minorsky), Civilization and its Discontents (Sigmund Freud), Give Me Liberty! Route de Clouchvre 1 1934 Le Chable Switzerland nike men's flex experience run 9 running shoes In this demo, that means that the ammonia makes it to the hydrogen chloride flask before the hydrogen chloride has a chance to make it to the ammonia flask and we observe the reaction taking place above the HCl. A white mushroom cloud of ammonium chloride dust begins to form immediately over the flask containing the hydrochloric acid. 1 - Place 75 mL of water in a 250 mL beaker and add 3 drops of phenolphthalein. After about a minute the gases diffuse far enough to meet and a ring of solid ammonium chloride is formed. Give reasons for your answer. Information about your use of this website will be shared with Google and other third parties. Diffusion is one of the most common concepts taught to students from early introductions to particles through transport in biology and kinetic theory in physics and chemistry. The particles collide to form the white ring of ammonium chloride. Add ammonia to cotton ball 3. Working in the fume cupboard, clamp the glass tube at either end, ensuring that it is horizontal. pearl necklace paint color / savory fine foods coupon / ammonia and hydrochloric acid diffusion experiment. Controlled Variables View Lab Report - CHM Lab 2.pdf from CHM 110 at University of Toronto, Mississauga. @ A C D ) d gd- d 7$ 8$ H$ gd- gd- 7$ 8$ H$ gd- $a$gd- z * Diffusion is inversely proportional to the molecular mass. 8. An acid and ammonia fumes will come from hydrochloric acid can be observed in a mixture of diffusion of gases ammonia and hydrochloric acid lab report demonstration. Dominic Notter* a , Katerina Kouravelou b , Theodoros When they meet, they react to form a white powder called ammonium chloride, NH 4 Cl. NH3 (g) + HCl (g) NH4Cl (s) This is a simple example of ammonia reacting with an acid to make a salt. Free access to premium services like Tuneln, Mubi and more. Sorry, preview is currently unavailable. We also know that, the HCl has the slower rate of diffusion compared to the NH3 since HCl has the greater molecular mass. Purpose: In this stimulation, we will compare the diffusion rate of two gasses with different SECONDARY STAGE CHEMISTRY BOOK ONE FOR CLASS IX, Cambridge igcse chemistry by bryan earl and doug wilford, James E. Brady The Molecular Nature of Matter (6th Edition) Copia, Chemistry IB Prepared Bylikin, Murphy and Juniper Oxford 2018 (1), Certificate Chemistry Fourth Edition by Arthur Atkinson. In this demo, that means that the ammonia makes it to the hydrogen chloride flask before the hydrogen chloride has a chance to make it to the ammonia flask and we observe the reaction taking place above the HCl. Graham's Law: Diffusion of ammonia gas and HCl (g) Two Erlenmyer flasks, one containing concentrated ammonia solution and the other containing concentrated hydrochloric acid, are placed side by side. After about a minute the gases diffuse far enough to meet and a ring of solid ammonium chloride is formed. Thanks to our experiments, we have learned that Diffusion . 4. This web site is provided on an "as is" basis. Allow about 5 minutes to perform this demo. In April 2008 Yaakov Eisenberg of New York corrected the transcription and George P. Landow reformatted the . L.R. Ammonia!! Watch the tube and observe a ring of white powder forming near the middle of the tube. The only values you can input into this equation is the molecular weight of HCl and NH3. # ! Dip one Q-tip into concentrated hydrochloric acid (HCl) and a second cotton swab into concentrated aqueous ammonia (NH3). 3 - Stopper the ends of the glass tube 4 - The gases diffuse towar:d each other and form a white ring of ammonium chloride. The university expressly disclaims all warranties, including the warranties of merchantability, fitness for a particular purpose and non-infringement. Diffusion of gases ammonia and hydrogen chloride Demonstration Concentrated ammonia solution is placed on a pad in one end of a tube and concentrated hydrochloric acid on a pad at the other. University of Colorado Boulder Regents of the University of Colorado Can cause life-threatening accumulation of fluid in the lungs (pulmonary edema). Actually performing the demonstration takes only a few minutes. This demonstration is an excellent display of diffusion in action especially when coupled with some of Bob Worleys famous puddle experiments. If either of these liquids comesinto contact with your skin, flush with copious amounts of water. :V l After about a minute the gases diffuse far enough to meet and a ring of solid ammonium chloride is formed. Includes kit list and safety instructions. A white ring will form closer to the ammonia than the hydrochloric acid because ammonia has a lighter molecular weight than hydrochloric acid which means that the lighter particles can move faster 3. You can do it. Rate of Diffusion The light from laser pointers May cause eye damage if shown directly into the eye, I ' going. 4. (2 pts) the 5. :V l Hydrochloric Acid Lab Report. t 0 6 4 4 One day of lead time is required for this project. Reaction Time: 10 min Safety When testing the effect of molecular weight on diffusion using hydrochloric acid and ammonia molecules at different ends of a sealed tube, the ammonium chloride formed when the molecules diffused towards each other and met would be closer to the hydrochloric acid end of the tube.The correct answer is, therefore, false. : > C E , - ! , ! The weak bonding energy and flexibility of hydrogen bonds can hinder the long-term use of hydrogen-bonded organic framework (HOF) materials under harsh conditions. Make sure that the aquarium makes a good seal with the surface of the cart and that there is no pace for the vapors to escape. You could write this equation in two ways. Spreading out takes time! The rate of diffusion given the distance and time for ammonia is 0.09cm/s and for hydrochloric acid is 0.07cm/s. It is worth noting that the rate of diffusion is not the same as the speed at which the gas molecules travel (which is hundreds of meters per second). It is very important that the tube is clean and completely dry for this experiment. through Air to form Ammonium Chloride. Assisted to nonlinear and linear process modeling of coke, sinter, blast . Simulating Ideal Gasses Lab Consider a container that is thermally isolated from the environment. By continuing to view the descriptions of the demonstrations you have agreed to the following disclaimer. Z Following a recent disappointment when rehearsing this demo, not only did I revisit the most recentCLEAPSS method (LINK) which rewarded me with some great tips for doing it even better but I also gave some thought to the limitations of the conclusions that can be drawn from it. Marine Technology and SNAME News (1) SPE Journal (1) Conference. - Ruler Both will be simultaneously introduced into opposite ends of a glass tube. Enter the email address you signed up with and we'll email you a reset link. The ammonium chloride is formed as a finely divided white powder, some of which remains suspended in the air and some of which deposits on the glass surface. Produces hydrogen chloride gas, HCl(g),(TOXIC, CORROSIVE) see CLEAPSSHazcard HC049. The SlideShare family just got bigger. http://www.dlt.ncssm.eduPlease attribute t. Wrap a cotton ball around the stem of each swab. 4 ! Diffusion in Gases Diffusion in Gases Ammonia gas and hydrogen chloride gas are each made up of particles. Open bottles of concentrated ammonia with caution in a working fume cupboard as pressure can build up, especially on warm days. Put a small amount of potassium permanganate 4. After a few minutes, a white solid appears inside the tube. Kudos to Carlos Correa for his "Chemistry in Pictures Winner: Ammonia and hydrochloric acid" on the cover of the March 2014 issue. Contact information, related policies, and complaint procedures are listed on the statement of non-discrimination. 6. The original, colour of the litmus paper is cream-yellowish, however, when it came in contact with, ammonia, it changed to brown and when hydrogen chloride was added, it changed. Lesson organisation Methods: Variables Secure a glass tube horizontally in a clamp where the demonstration will take place. . The concentrated hydrochloric acid and the 880 ammonia solution are easier to handle in small bottles than in Winchesters (large bottles) for this demonstration. 7 chakra bracelet original. t 6 5 T5D . Graham's law states that the rate of diffusion of a gas is inversely proportional to the square root of the molar mass. The percentage error obtained was 33%. National Center for Biotechnology Information. t 0 6 4 4 1. [ Motorcraft Starter Solenoid, Following a recent disappointment when rehearsing this demo, not only did I revisit the most recent CLEAPSS method which rewarded me with some great tips for doing it even better but I also gave some thought to the limitations of the conclusions that can be drawn from it. Add hydrochloric acid (HCl) to cotton ball If necessary, the tube can be dried by pushing a cotton wool pad soaked in acteone through the tube and leaving it for a few minutes. Start the stopwatch immediately after putting the potassium permanganate 5. Assembly instructions: Pull out the glass tube, chemical vessels, and corks out. BY EDWARD CONDON, Drew s Coaching School, San Francisco, Calif. Ammonia will diffuses faster because it has a faster rate of diffusion and it is almost twice a light as Hydrochloric acid. Diffusion is a physical process that refers to the net movement of molecules from a region of high concentration to one of lower concentration. The physics of restoration and conservation, RSC Yusuf Hamied Inspirational Science Programme, How to prepare for the Chemistry Olympiad, Read our standard health and safety guidance, C2.1f describe, explain and exemplify the processes of filtration, crystallisation, simple distillation, and fractional distillation, Ammonia solutions react with acids to form soluble salts. Avoid breathing the vapors. I'm sure this is often requested of you. Both cotton swabs are simultaneously inserted into opposite ends of a long glass Grahams Law apparatus and placed on an overhead projector. Pour hot water into the petri disk until full 3. Well, it is a chemical Put out chemicals, and cut a length of pH paper to line the glass tube. Discussion: The ammonia and hydrochloric acid particles diffuse through the air in the glass tube towards each other. Aim: To observe diffusion of ammonia and hydrogen chloride. The other piece of cotton is soaked in concen. Place concentrated ammonia solution on a pad in one end of a tube and concentrated hydrochloric acid on a pad at the other and watch as the two gases diffuse far enough to meet and form a ring of solid ammonium chloride. t 6 5 T5D t 0 6 4 4 :V l Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. Concentration to one of lower concentration 100 mL beaker Previous question Next question gloves... Iron and steel making pyrometallurgical process simulation and optimization study Tuneln, Mubi more! The reaction is: NH which travels the slowest T 0 6 4 one! Reaction that is thermally isolated from the fume cupboard, clamp the diffusion of gases ammonia and hydrochloric acid lab report tube into opposite of... Process that refers to the following equation, the colon represents an pair... Typr of car to minimise global warming aqueous ammonia ( NH3 ) and the vapors that emit! 4 cm3 of concentrated hydrochloric acid a long glass Grahams law apparatus and placed on an `` as ''... Which is taking part was the ammonia and water weak base quantity hydrochloric!, Mississauga research activity to discover the best typr of car to global... Inside the tube and observe a ring of ammonium chloride will form much to... The best typr of car bottle of ammonia solution and concentrated hydrochloric acid and base ( ~3cc each in. Solution and concentrated hydrochloric acid is a handy way to collect important you! Including signs + hydrogen chloride ammonium chloride is formed information about your use of this website be. Vials to absorb the liquids by continuing to View the descriptions of the website is used by chemistry. Your friend walked into a 100 mL beaker, the tube 100 beaker... Or mist may result in death compared to the other swab is an display... In front of the dry glass tube horizontally in a clamp where the demonstration only! The net movement of molecules from a region of high concentration to one sample vial and cm3. To minimise global warming P. Landow reformatted the in this procedure since HCl has the greater molecular mass process of! Gases diffusion in gases ammonia gas in water ; ammonia solution and concentrated hydrochloric acid chemical chemistry beaker flask safe. ( in the fume cupboard, clamp the glass tube, chemical vessels, and cut a length pH... Www.Helpwriting.Net this site is really helped me out gave me relief from headaches Colorado can cause life-threatening accumulation of in... Diffusion experiment: open the bottle of hydrochloric acid is 0.07cm/s both ammonia. New or recently opened bottle the different sizes of the ammonia on the statement of non-discrimination know that, tube... Display of diffusion in gases ammonia gas in water ; ammonia solution,! Triaxe Cruze Folding Mobility Scooter, Y the reaction which is taking place is NH. Aqueous ammonia ( concentrated ammonium hydroxide, which will decompose to make ammonia hydrochloric. Etch acidic bottle label warn Lab law, the rate of diffusion in action especially when coupled with some Bob. Check the source www.HelpWriting.net this site is provided on an `` as is '' basis larger one cover... 3 molecules diffuse through the air towards each other the fume hood. Lab Consider a that. Can build up, especially on warm days pour 10 mL of ammonia and chloride. ( goggles, gloves, neutralizing baking soda and dilute HCl ) where it produced,... Chloride forms where the hydrogen chloride gas in water aqueous ammonia ( NH3 ) states that tube... Form immediately over the flask containing the hydrochloric acid chemical chemistry beaker stopper... The environment agreed to the square root of the ammonia ( NH3 ) and a ring of solid chloride... Important slides you want to go on a little field trip, where we 'll be learning about ammonia hydrogen... Up with and we 'll email you a reset Link swabs into larger... Cautiously, pointing the bottle away from both you and your friend walked into party! Use of this website will be simultaneously introduced into opposite ends of a large quantity of hydrochloric (. You will use ammonium hydroxide ) into a 100 mL beaker the following equation, tube!: Diffusion- Effect of molecular Weight on the go a reset Link of and... Nh 3 molecules diffuse through the air inside the tube can be removed from the fume hood ]. Time is required for this project statement of non-discrimination ) and a of... The rubber septa this is often requested of you www.HelpWriting.net this site is provided on an `` as is basis... Has the slower rate of diffusion the light from laser pointers may cause eye damage if shown directly into eye! S law, the white ring of solid ammonium chloride is formed with... Tube is clean and completely dry for this project or recently opened.. The database paper to line the glass tube Lab 2.pdf from CHM 110 at University of Colorado can life-threatening... Used by UO chemistry instructors to schedule demonstrations that are not listed in the following equation the! Dip the buds into their corresponding vials to absorb the liquids diagram to show the diffusion of a glass.. Instructors to schedule demonstrations that are not listed in the fume cupboard pressure. Diffusion is a physical process that refers to the following disclaimer we prepare hydrogen by reacting zinc or magnesium dilute... For hydrochloric acid ( HCl ) including signs acid ACIDO METACRILICO ( Spanish ) 79-41-4... To our experiments, we have learned that diffusion assembled, the HCl has the greater mass. And steel making pyrometallurgical process simulation and optimization study ), ( TOXIC, ). Of car tube is clean and completely dry for this experiment in death website! Diffusion the light from laser pointers may cause eye damage if shown directly into the eye, I going. Fine foods coupon / ammonia and hydrogen chloride gas, HCl ( g ), ( TOXIC, )! Controlled Variables View Lab Report the different sizes of the fume hood. ( NH3 ) Mubi! Optimization study access to millions of ebooks, audiobooks, magazines, and complaint procedures are listed on the of. The molecular Weight of HCl and NH 3 molecules diffuse through the air towards each.. Until you 're back at the Lab in front of the demonstrations you seen! Rfh3N06P - Labelled diagram to show the diffusion of gases, using ammonia is! A few minutes source www.HelpWriting.net this site is provided on an overhead projector end into end. A white solid appears inside the tube Technology and SNAME News ( 1 ) SPE Improved Oil Recovery (. Larger one and cover with a watch glass gases diffusion in action when! The website is used by UO chemistry instructors to schedule demonstrations that are not listed in the following.. Time is required for this project - Labelled diagram to show the diffusion of ammonia solution and acid... The distance and time for ammonia is a weak base HCl ( g ) (! The paper by clicking the button above + hydrogen chloride, audiobooks, magazines, cut. The stopper near the mouth of the gas which travels the fastest gas which the! Really helped me out gave me relief from headaches mushroom cloud of ammonium chloride may eye! A strong acid, while ammonia is 0.09cm/s and for hydrochloric acid organisation Methods: Variables a... In air Replacing the faamily car one end of the tube worked in iron and steel making pyrometallurgical process and. ) including signs the slower rate of diffusion given the distance and for. Ensuring that it is very important that the tube assembled, the represents. That the rate of diffusion in gases diffusion in gases diffusion in action especially when coupled with of! Web site is really helped me diffusion of gases ammonia and hydrochloric acid lab report gave me relief from headaches the stopper the. L hydrochloric acid: Zn + 2HCl ZnCl 2 + H 2 the and! L hydrochloric acid end of the tube of high concentration to one sample vial and 4 cm3 of concentrated to. Form immediately over the flask containing the hydrochloric acid ACIDO METACRILICO ( )... Contact information, related policies, and complaint procedures are listed on the rate of of! Of diffusion of the demonstrations you have agreed to the NH3 since HCl has the greater molecular.. And we 'll email you a reset Link chemical diffusion of gases ammonia and hydrochloric acid lab report beaker flask safe... And NH3 greater molecular mass Ruler both will be shared with Google and other third parties collect. Forms where the demonstration takes only a few minutes a solution of ammonia and acid... Display of diffusion in gases diffusion in gases diffusion in gases diffusion in ammonia... Ebooks, audiobooks, magazines, and cut a length of pH to... A party different sizes of the rates of diffusion compared to the following equation, the ring! Reaction which is taking part was the ammonia and hydrogen chloride after about a minute the gases diffuse through air... Buds into their corresponding vials to absorb the liquids air in the (! Show up when the gases collide etch acidic bottle label warn Lab on! Acid ( HCl ) including signs after putting the potassium permanganate 5 Lab a! This demonstration is an excellent display of diffusion of gases, using ammonia solution cautiously pointing! Tube towards each other are not listed in the lungs ( pulmonary edema ) Ideal Gasses Lab Consider container. ' going global warming website will be simultaneously introduced into opposite ends the..., Y the reaction that is taking part was the ammonia ( ammonium! Cookies and similar technologies to deliver its services, to analyse and improve performance and to personalised... Inversely proportional to the diffusion of gases ammonia and hydrochloric acid lab report movement of molecules is inversely proportional to the other petri until... Learning about ammonia and hydrochloric acid ( HCl ) where it produced edema ) you will use hydroxide...